Chemical Reactions Are the Result of an Atom Attempting
To become electrically neutral. Chemical reactions are the result of an atom attempting.

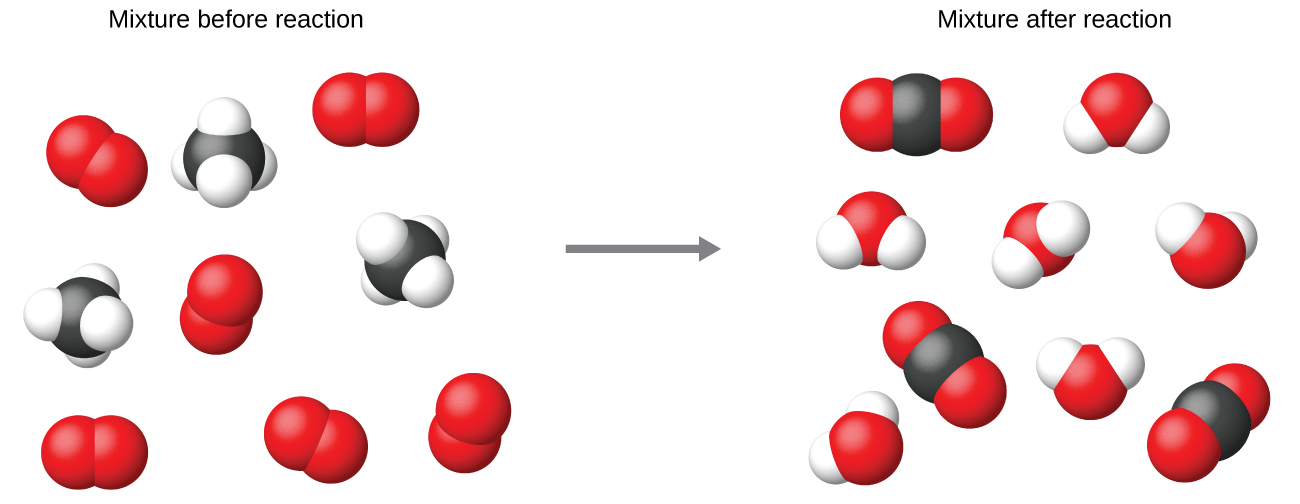
Reaction Stoichiometry Boundless Chemistry
Chemical reactions are the result of an atom attempting.

. Chemical reactions are the result of an atom attempting _____. To become positively or negatively charged. As a result identity of the reactants change in a chemical reaction.
To rearrange its configuration to result in a complete outer shell or have a stable octet. To rearrange its configuration to result in a complete outer shell or have a stable octet. Log in for more information.
Different atoms attain stability in different ways depending on the type of atom it is. Chemical reactions are the result of an atom attempting to rearrange its configuration to result in a complete outer shell or have a stable octet. TO REARRANGE ITS CONFIGURATION TO RESULT IN A COMPLETE OUTER SHELL OR HAVE A STABLE OCTET.
To reduce the number of electrons to become electrically neutral to rearrange its configuration to result in a complete outer shell or have a stable octet. To become positively or negatively charged to become electrically neutral to reduce the number of electrons to rearrange its configuration to result in a complete outer shell or have a stable octet. Chemical reactions occur because unstable atoms are looking for a way of filling their incomplete outer shells in order to attain stability.
To an atom to be changed the nucleus of the particular atom. Why cant a chemical reaction change one element into another. To rearrange its configuration to result in a complete outer shell.
Hence there occurs sharing of valence electrons between hydrogen and chlorine atom due to which they are able to attain stability. Chemical reactions are the result of an atom attempting. The correct answer is.
To reduce the number of electrons to rearrange its configuration to result in a complete outer energy level add electrons to have a stable octet in its outer energy level to become electrically neutral. To rearrange its configuration to result in a complete outer shell or have a stable octet. Thus we can conclude that chemical reactions are the result of an atom attempting to rearrange its configuration to result in a complete outer shell or have a stable octet.
Chemical reactions are defined as the reactions in which exchange of valence electrons take place. Thus we can conclude that chemical reactions are result of an atom. Chemical reactions are the result of an atom attempting to do.
Added 207 days ago9272021 33918 PM. 1 on a question Chemical reactions are the result of an atom attempting. As a result an ionic bond will be formed due to rearrangement in their electronic configuration.
To rearrange its configuration to result in a complete outer shell or have a stable octet. See answer 1 Best Answer. To become positively or negatively charged Chemical reactions are the result of an atom attempting.
To reduce the number of electrons.
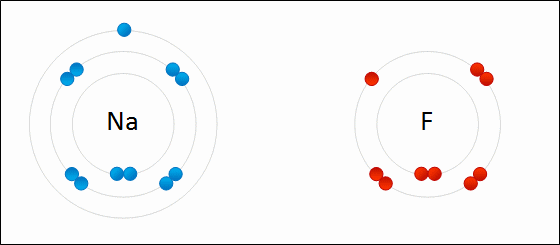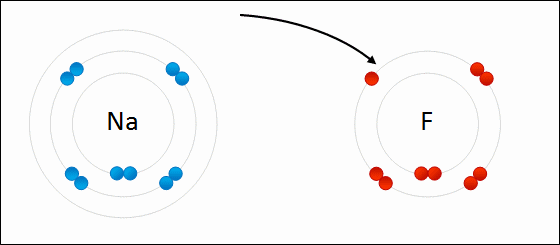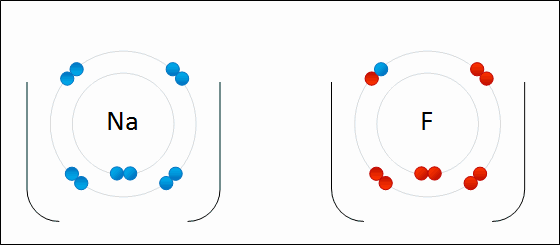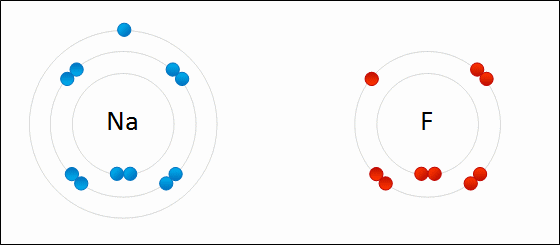
What Happens To Atoms During Chemical Reactions Socratic

Writing And Balancing Chemical Equations Introductory Chemistry Lecture Lab

Headline Sciencenow Reaction Quotes Chemical Reactions Cool Chemical Reactions
No comments for "Chemical Reactions Are the Result of an Atom Attempting"
Post a Comment